-- 2019 Net Revenues Increased 38% over 2018 --
-- Restated Annual Financial Results for 2018 Also Reported --
TORRANCE, Calif., Jan. 25, 2021 /PRNewswire/ -- Emmaus Life Sciences, Inc. (OTC: EMMA), a leader in the treatment of sickle cell disease, reported today its financial results for the year ended December 31, 2019 and its restated financial results for the year ended December 31, 2018. As previously disclosed in the Current Report on Form 8-K filed with the Securities and Exchange Commission ("SEC") on July 8, 2020, the board of directors of Emmaus Life Sciences, Inc. ("Emmaus" or the "Company"), based on the recommendation of the audit committee concluded, that due to errors identified in the previously issued financial statements for the year ended December 31, 2018 as well as the previously filed unaudited consolidated financial statements for the three and nine months ended September 30, 2019, the Company would restate the previously issued financial statements.
"We are pleased to share our strong financial results for 2019 and our restated financials for 2018 with our current and prospective stakeholders. We look forward to filing our 2020 10-Qs and communicating our financial results for the three months ended March 31, June 30 and September 30, 2020 as soon as possible," said Dr. Yutaka Niihara, M.D., M.P.H., Chairman and Chief Executive Officer. "In advance of the quarterly filings, Emmaus also appreciates the opportunity to provide a summary of our business progress."
Financial Results for the Year Ended December 31, 2019
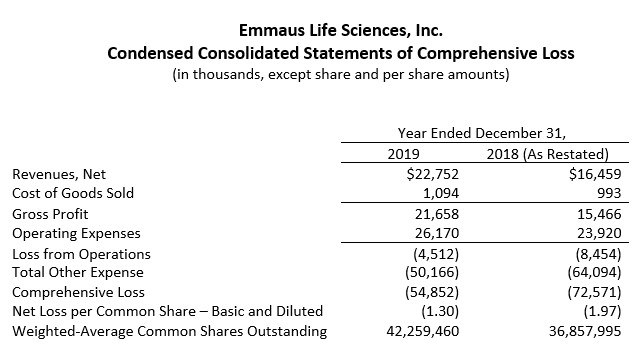
Net revenues for 2019 were $22.8 million compared to $16.5 million in 2018, an increase of 38%. This significant growth was driven primarily by the on-going roll-out and market acceptance of Endari® resulting in increased sales to the Company's customers that include the nation's leading pharmaceutical distributors, specialty pharmacies and physician group purchasing organizations. Emmaus believes it is well positioned for continued growth as it expands the commercialization of Endari® in the U.S. and implements its early access programs and commences marketing outside the U.S.
Operating expenses were $26.2 million in 2019, compared to $23.9 million in 2018. Of the $17.0 million in general and administrative expenses, $2.4 million was attributable to one-time, non-recurring expenses in connection with the merger transaction completed on July 17, 2019 (the "Merger"). The $2.3 million increase in selling expenses to $7.0 million in 2019 was to support the commercialization of Endari® and the related increase in net revenues. The $0.5 million increase in research and development expenses to $2.2 million in 2019 was attributable to the Pilot/Phase 1 study of the Company's prescription grade L-glutamine oral powder to treat diverticulosis.
Operating loss for 2019 was $4.5 million, compared to an operating loss of $8.5 million in 2018. When adjusted for the non-recurring general and administrative expenses relating to the Merger, the operating loss for 2019 was $2.1 million.
"2019 saw Emmaus continue to make progress in the U.S. commercialization and roll-out of Endari as seen in our 38% growth in net revenues over 2018 and greatly improved overall financial results. Our operating loss was reduced significantly in 2019 and our balance sheet was strengthened through the merger transaction that closed in July," added Dr. Niihara. "Importantly, we have made significant advances in our primary goal of ensuring that every medically appropriate sickle cell disease patient has access to Endari on a cost-effective and timely basis."
Business and Other Company Updates
Direct Sales Force - Effective January 1, 2020, Emmaus switched from the use of a contract sales organization to its own direct sales force and the Company continues to build its internal sales and marketing capabilities. Emmaus currently has 23 employees in its sales and marketing department.
Middle East and North Africa ("MENA") Region - Emmaus continues to make progress in developing markets for Endari® in the MENA region. On June 29, 2020 Emmaus announced receipt of Endari® marketing authorization from the Israeli Ministry of Health and on July 23, 2020 announced the opening of its Dubai office. In addition, on November 10, 2020 the Company announced its submission of a temporary license application in Bahrain for Endari®. These developments will accelerate the Company's efforts to reach the estimated 100,000 potentially treatable sickle cell disease patients in the MENA region.
Diverticulosis Study - The Company's Pilot/Phase 1 study of the same prescription grade L-glutamine oral powder used in Endari® in treating diverticulosis commenced in April 2019 and is ongoing. The COVID-19 pandemic has slowed the progress of clinical trials in the pharmaceutical industry, in general, and patient enrollment at one of the three Emmaus trial sites was suspended temporarily. Nonetheless, patient enrollment was completed, and Emmaus is confident the study will ultimately evaluate the change in the number and size of colonic diverticula and assess safety in patients with diverticulosis. Limited interim study results to date have been encouraging, suggesting that Endari® may be effective in slowing and reversing the progression of diverticulosis.
Manufacturing - The COVID-19 pandemic has not interrupted the Company's supply chain and Emmaus has sufficient inventory of Endari® to meet current and projected patient needs and support ongoing clinical trials. Progress continues at the manufacturing facility in Ube, Japan purchased by a 40% owned investee of Emmaus in December of 2019. To meet the long-term potential demand for prescription grade L-glutamine, Emmaus, its partners and contractors are in the process of obtaining regulatory approvals and recertifications of the facility. The Company currently anticipates that test production will commence in early 2021 with regulatory approval expected in 2022.
Endari® Support Program - Emmaus announced the launch of the Endari® Support Program on December 8, 2020 to provide patients who are unable to afford Endari® access to the medication for minimal or no cost. For more information, please see www.EndariRx.com/ESP.
Endari® Label Change - On October 27, 2020, Emmaus announced that the FDA approved an updated label for Endari® to better inform healthcare professionals and their sickle cell disease patients. The updated label includes a statement that the clinical benefits of Endari® were observed irrespective of hydroxyurea use, thereby supporting the use of Endari® as a monotherapy or in combination with hydroxyurea as important treatment options for sickle cell disease patients.
Michigan Revises Prior Authorization Criteria for Endari® - The Michigan Department of Health and Human Services ("MDHHS") notified Emmaus that, effective January 1, 2021, the following changes will be made regarding the initial authorization of Endari® thereby allowing it to be prescribed to more of Michigan's sickle cell disease patients, more quickly, than under the prior authorization criteria: (i) the history of hydroxyurea use and adherence or intolerance/contraindication to hydroxyurea will be eliminated from the Endari® initial authorization documentation requirements and (ii) "patient/family refusal" will be added to the existing justifications of intolerance or contraindication to the use of hydroxyurea. With this recent revision, MDHHS joins many other state health and human services agencies in eliminating the prior use of hydroxyurea as a requirement for the initial authorization of Endari® for the treatment of sickle cell disease.
COVID-19 Impact - Emmaus is encouraged that patient compliance and adherence as well as health monitoring have held up well in the wake of the COVID-19 pandemic, which may bode well for improved patient adherence when the pandemic subsides. However, ongoing stay-at-home orders and business lockdowns may adversely affect the Company's future revenues, results of operations and financial condition, and management will continue to monitor COVID-19 developments and take necessary actions to minimize any impact on the Company's business.
Trading and Quotation of the Company's Common Stock - On July 30, 2020, Emmaus was notified by the OTC Markets Group, Inc. that its common stock would no longer be eligible for quotation on the OTCQB tier as of the open of the market on August 3, 2020 due to the delays in filing the company's Annual Report on Form 10-K for 2019 and Quarterly Reports on Form 10-Q for the quarters ended March 31, June 30, and September 30, 2020. Once the Company has filed with the SEC its 10-K for 2019 and 10-Qs for March 31, June 30, and September 30, 2020, posted the OTCQB Certification and verified the Company profile through OTCIQ.com, the OTC Markets Group, Inc. will review the Company to ensure that it still meets all the OTCQB Standards at that time. If no further items are needed, the Company's common stock will be moved back to the OTCQB tier beginning the next trading day. In the meantime, quotes will continue to be available on the OTC Pink tier.
2020 10-K and Annual Shareholders Meeting - The Company intends to file its 2020 Annual Report on Form 10-K with the SEC in or about March 2021 and hold its next Annual Stockholders Meeting for the election of directors and to review 2020 operating results as soon as practicable thereafter.
About Emmaus Life Sciences
Emmaus Life Sciences, Inc. is a commercial-stage biopharmaceutical company engaged in the discovery, development, marketing and sale of innovative treatments and therapies, including those in the rare and orphan disease categories. For more information, please visit www.emmausmedical.com.
About Endari® (prescription grade L-glutamine oral powder)
Indication (U.S.) - Endari® is indicated to reduce the acute complications of sickle cell disease in adult and pediatric patients five years of age and older.
Important Safety Information
The most common adverse reactions (incidence >10 percent) in clinical studies were constipation, nausea, headache, abdominal pain, cough, pain in extremities, back pain, and chest pain.
Adverse reactions leading to treatment discontinuation included one case each of hypersplenism, abdominal pain, dyspepsia, burning sensation, and hot flash.
The safety and efficacy of Endari® in pediatric patients with sickle cell disease younger than five years of age has not been established.
For more information, please see full Prescribing Information of Endari® at: www.EndariRx.com/PI.
About Sickle Cell Disease
Sickle cell disease is an inherited blood disorder characterized by the production of an altered form of hemoglobin which polymerizes and becomes fibrous, causing red blood cells to become rigid and change form so that they appear sickle shaped instead of soft and rounded. Patients with sickle cell disease suffer from debilitating episodes of sickle cell crises, which occur when the rigid, adhesive and inflexible red blood cells occlude blood vessels. Sickle cell crises cause excruciating pain as a result of insufficient oxygen being delivered to tissue, referred to as tissue ischemia, and inflammation. These events may lead to organ damage, stroke, pulmonary complications, skin ulceration, infection and a variety of other adverse outcomes. Sickle cell disease is a significant unmet medical need, affecting approximately one hundred thousand patients in the U.S. and millions worldwide, the majority of which are of African descent. An estimated 1-in-365 African American children are born with sickle cell disease.
Forward-looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding the Company's business and operations and future financial results. These forward-looking statements are subject to numerous assumptions, risks and uncertainties which change over time, including uncertainties related to Emmaus' working capital and ability to continue as a going concern and obtain needed financing and other risk factors disclosed in the Company's 2019 Annual Report on Form 10-K and other reports filed with the SEC, and actual results may differ materially. Such forward-looking statements speak only as of the date they are made, and Emmaus assumes no duty to update them, except as may be required by law.
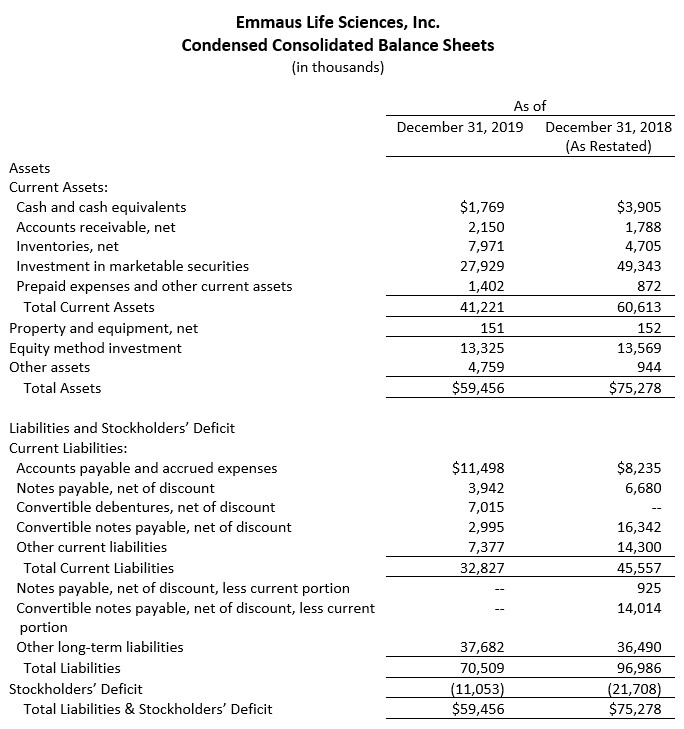
(Selected Consolidated Financial Data Follows)
SOURCE Emmaus Life Sciences, Inc.